Understanding Points
Understanding Points
A1.1.1 Water as the medium for life
A1.1.2 Hydrogen bonds as a consequence of the polar covalent bonds within water molecules
A1.1.3 Cohesion of water molecules due to hydrogen bonding and consequences for organisms
A1.1.4 Adhesion of water to materials that are polar or charged and impacts for organisms
A1.1.5 Solvent properties of water linked to its role as a medium for metabolism and for transport in plants and animals
A1.1.6 Physical properties of water and the consequences for animals in aquatic habitats
A1.1.7 Extraplanetary origin of water on Earth and reasons for its retention (HL only)
A1.1.8 The relationship between the search for extraterrestrial life and the presence of water (HL only) |
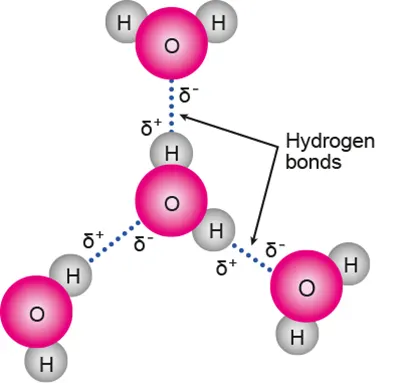
Structure of water
•
Polar covalent bonds: unequal sharing of e⁻ → partially negative O atom, partially positive H atoms
•
Hydrogen bonding: intermolecular force between the O and H of different H₂O molecules
Properties of water
Property
Cohesion
Definition
Binding of two like molecules
Water to water
Due to hydrogen bonding
Example: Capillary action
Adhesion
Binding of two different molecules
Water to other polar molecules
Example: Water adheres to cellulose in cell walls. Together, cohesion and adhesion enable water transport through the xylem
Surface tension
Cohesion in water creates an elastic membrane at the surface
Example: Water striders use water surfaces as a habitat
Solvent property
H bonding or polar bonds dissolve polar substances in water. This makes water a good transport medium and chemical reaction site
Example: Blood plasma transports minerals, 𝛼𝛼, proteins, other polar substances
Thermal property
High specific heat capacity: a large amount of heat is required to raise the temperature of 1 g of water by 1℃
High latent heat of evaporation: a large amount of heat is needed to convert 1 g of liquid water to gas at constant temp.
Example: Stable aquatic environment. Heat transfer in blood. Sweat is an evaporative coolant in thermoregulation
Transparency
Light can penetrate water
Example: Aquatic plants can carry out photosynthesis
Density
Water has a higher density than ice
Example: In the winter, ice forms from the top of the water, so a stable environment for aquatic organisms is maintained
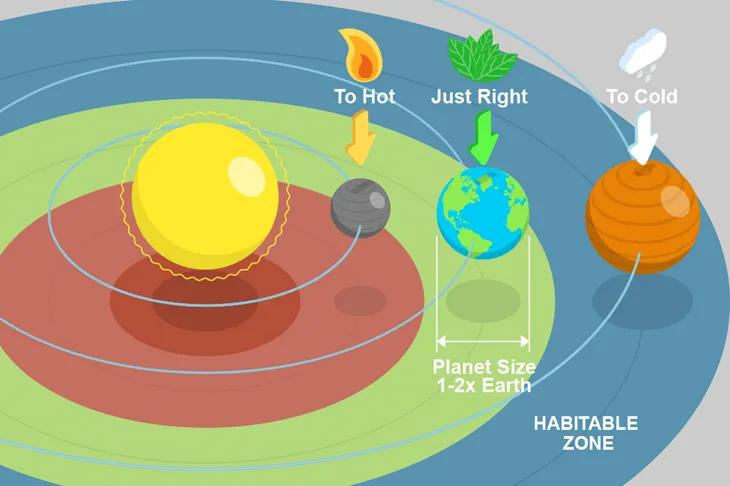
*(AHL) The origin of water and extraterrestrial life
•
Water on Earth is hypothesized to have come from asteroids
•
Water was retained due to Earth’s gravity and relatively cool temp. that prevented evaporation
•
Goldilocks zone: habitable zone around a star, where water can exist in liquid state