1.1 Introduction to the particulate nature of matter
Understanding Points |
Structure 1.1.1—Elements are the primary constituents of matter, which cannot be chemically broken down into simpler substances.
Compounds consist of atoms of different elements chemically bonded together in a fixed ratio. Mixtures contain more than one element or compound in no fixed ratio, which are not chemically bonded and so can be separated by physical methods. |
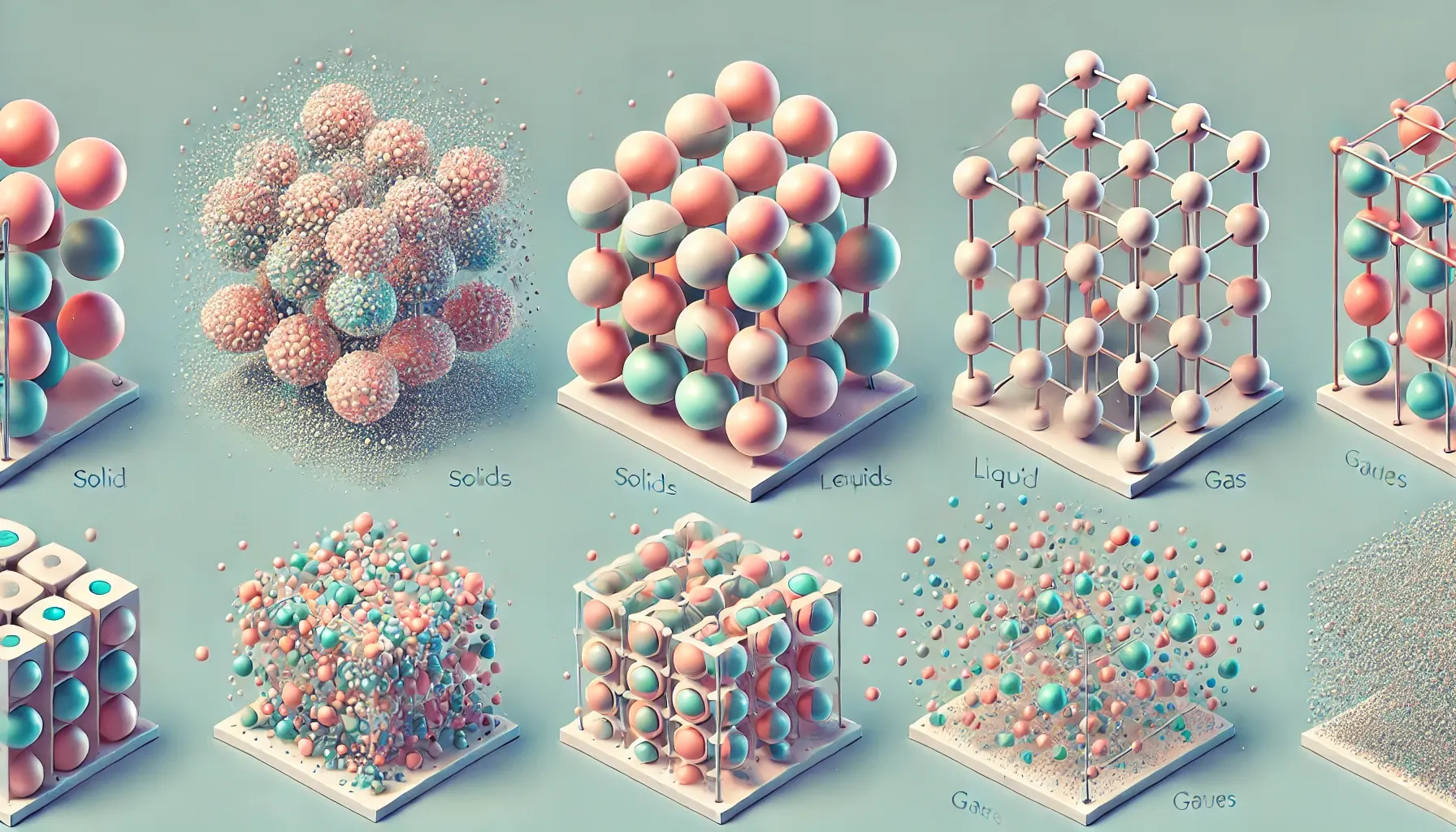
Structure 1.1.2—The kinetic molecular theory is a model to explain physical properties of matter
(solids, liquids and gases) and changes of state. |
Structure 1.1.3—The temperature, T, in Kelvin (K) is a measure of average kinetic energy Ek of
particles. |
Atom: basic building block of matter that can neither be created nor destroyed
•
Described in Dalton's Atomic Theory
Element: a chemical species that cannot be further decomposed by chemical reaction
•
Elements have the same number of protons in their nuclei
•
A sample of an element contains only one type of atom in the whole sample
•
Not all elements are monatomic: diatomic elements are Br2, I2, N2, Cl2, H2, O2, F2
Compound: a pure substance composed of two or more elements bonded chemically
•
A compound can be broken down chemically but not physically
•
For a given compound, the elements are in fixed ratios → law of definite proportion
◦
e.g. Na2O; Na:O = 2:1
•
A compound has different physical and chemical properties from its constituent elements
◦
e.g. Na, O2, and Na2O have very different chemical and physical properties
Mixture: two or more substances (elements, compounds) that are not chemically bonded
•
General properties:
◦
The components of a mixture can be easily separated (relatively)
◦
The chemical or physical properties of the components do not change
•
Examples:
◦
Air: mixture of gases (N2, O2, etc.)
◦
Table salt dissolved in water (NaCl and H2O)
◦
Oil and water
•
Classification:
◦
Homogeneous mixture: substances are evenly distributed
▪
Examples: air, table salt in water, alloys, solutions (soluble solid in liquid)
▪
◦
Heterogeneous mixture: substances are unevenly distributed
▪
Examples: oil and water, suspensions (insoluble solid in liquid)
▪
Separation of mixtures
Physical properties of matter
Solid | Liquid | Gas |
• Has a definite shape and volume
• Cannot be compressed
• Low energy, tightly packed together | • Has a definite volume but not shape
• Cannot be compressed
• Slightly packed together in no specific pattern | • Has neither a definite volume nor shape
• Can be compressed
• High energy, freely moving |
The state of the element/compound can be written using state symbols
•
s= solid, l=liquid, g=gas, aq=aqueous (dissolved in water)
Physical Change | Name | Equation |
Solid → Liquid | Melting | A(s) → A(l) |
Solid → Gas | Sublimation | A(s) → A(g) |
Liquid → Solid | Freezing | A(l) → A(s) |
Liquid → Gas | Vaporization/boiling/
evaporation | A(l) → A(g) |
Gas → Solid | Deposition | A(g) → A(s) |
Gas → Liquid | Condensation | A(g) → A(l) |
•
Dissolving, A(s or l or g) → A(aq) is also a physical change but not a state change
Figure 1.1.1 Phase transitions of water
•
State change involves no change in temperature ∵ all energy gained or lost is used to break or make bonds
Maxwell-Boltzmann distribution
•
Shows the distribution of kinetic energy of particles
•
The area under the curve is the same as the number of particles does not change
•
At higher temperatures, more particles have a higher velocity and thus higher KE
•
T1 < T2
1.2 The nuclear atom (AHL)
Understanding points
Structure 1.2.3—Mass spectra are used to determine the relative atomic masses of elements from their isotopic composition. (AHL)
Mass Spectrometry: used to accurately determine the atomic or molecular mass of an isotope or molecule fragment
◦
5 Steps:
1.
Vaporisation
2.
Ionization
3.
Acceleration
4.
Deflection
5.
Detection
◦
Higher m/z (mass/charge) ratio = less deflection; lower m/z ratio = more deflection
◦
Example of a Mass Spectra (Boron)
▪
Relative atomic mass: weighted average of the mass of naturally occurring isotopes* of an atom compared on a scale relative to 12C.
◦
One relative atomic mass unit (amu)* is 1/12th of the mass of 12C.
◦
Using the relative abundance determined by the mass spectrum, the weighted average for Boron can be calculated:
▪
Ar=∑ (mass of isotope)(relative abundance of isotope)
▪
= (10 × 19.9%) + (11 × 80.1%) = 10.801