Understanding points
Understanding points
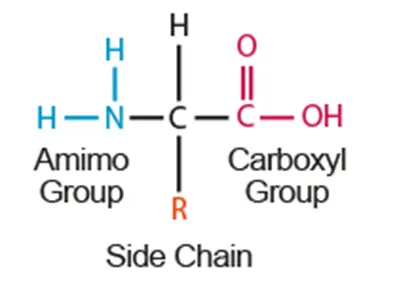
B1.2.1 Generalized structure of an amino acid
B1.2.2 Condensation reactions forming dipeptides and longer chains of amino acids
B1.2.3 Dietary requirements for amino acids
B1.2.4 Infinite variety of possible peptide chains
B1.2.5 Effect of pH and temperature on protein structure
B1.2.6 Chemical diversity in the R-groups of amino acids as a basis for the immense diversity in protein form and function (HL only)
B1.2.7 Impact of primary structure on the conformation of proteins (HL only)
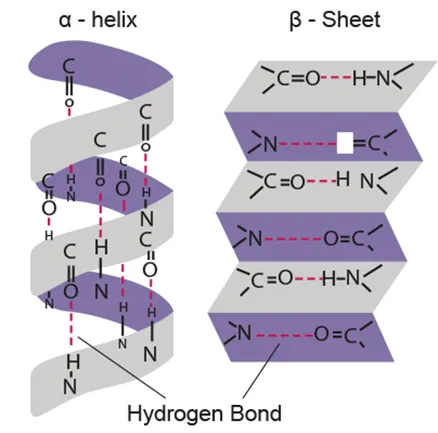
B1.2.8 Pleating and coiling of secondary structure of proteins (HL only)
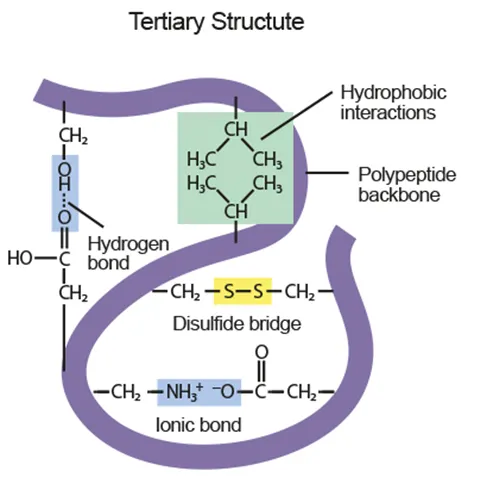
B1.2.9 Dependence of tertiary structure on hydrogen bonds, ionic bonds, disulfide covalent bonds and hydrophobic interactions (HL only)
B1.2.10 Effect of polar and non-polar amino acids on tertiary structure of proteins (HL only)
B1.2.11 Quaternary structure of non-conjugated and conjugated proteins (HL only)
B1.2.12 Relationship of form and function in globular and fibrous proteins (HL only) |
Amino acids
•
20 different types of naturally occurring 𝛼𝛼
•
*(AHL) R groups can be hydrophobic, hydrophilic, or charged
Denaturation
•
A structural change in a protein that results in the loss of its biological function
•
Heat and acid disrupt the intermolecular bonds in proteins
*(AHL)
Protein structure
Primary
Linear sequence of amino acids connected by peptide bonds
Covalent (peptide bond)
Secondary
α-helix (turned upon themselves) and β-pleated sheet (folded)
O-H bond between αα (Amine and Carboxyl)
Tertiary
Folding of secondary protein into 3D structure via side chain interactions
Covalent bonds: disulfide bridges
Intermolecular attractions : H bonds, ionic bonds, nonpolar-hydrophobic attractions
Quaternary
Two or more polypeptide chains linked together (with prosthetic group)
Protein form and function
Globular | Fibrous |
Round shaped, generally soluble in water
Functional role: enzymes and antibodies | Flat and narrow, insoluble in water
Structural role: keratin and collagen |