Understanding points
Understanding points
B1.1.1 Chemical properties of a carbon atom allowing for the formation of diverse compounds upon which life is based
B1.1.2 Production of macromolecules by condensation reactions that link monomers to form a polymer
B1.1.3 Digestion of polymers into monomers by hydrolysis reactions
B1.1.4 Form and function of monosaccharides
B1.1.5 Polysaccharides as energy storage compounds
B1.1.6 Structure of cellulose related to its function as a structural polysaccharide in plants
B1.1.7 Role of glycoproteins in cell–cell recognition
B1.1.8 Hydrophobic properties of lipids
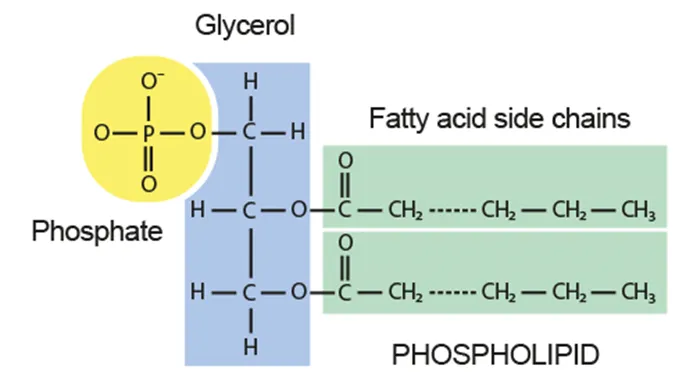
B1.1.9 Formation of triglycerides and phospholipids by condensation reactions
B1.1.10 Difference between saturated, monounsaturated and polyunsaturated fatty acids
B1.1.11 Triglycerides in adipose tissues for energy storage and thermal insulation
B1.1.12 Formation of phospholipid bilayers as a consequence of the hydrophobic and hydrophilic regions
B1.1.13 Ability of non-polar steroids to pass through the phospholipid bilayer |
Carbon as the basis of life
•
Forms strong covalent bonds with other atoms to form stable molecules
•
Can form up to 4 covalent bonds and give rise to molecules with complex structures
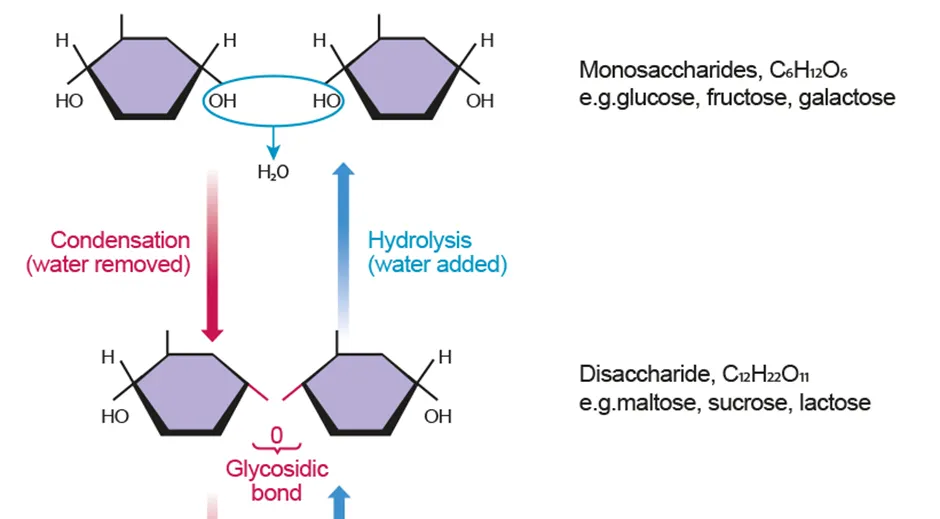
Condensation and hydrolysis
•
Condensation: bonds monomers together into a polymer by removal of water
•
Hydrolysis: breaking a polymer apart into smaller ones by addition of water
Carbohydrates
Monosaccharides
Glucose, fructose, galactose
Disaccharides
Sucrose, lactose, maltose
Oligosaccharides
A short chain of monosaccharides joined by glycosidic bonds
Polysaccharides
Starch: Amylose (α-1,4) and Amylopectin (α-1,4 & α-1,6)
•
Polymer of α-glucose, energy storage in plants
Glycogen: α-1,4 & α-1,6 linkages
•
Highly branched polymer of α-glucose, energy storage in animals
Cellulose: β-1,4 linkage
•
Straight chain, component of plant cell walls
Glycoproteins
Protein + oligosaccharide
•
Cell-to-cell recognition
•
Identification of self vs. foreign cells
•
Type A, B, O blood groups: different glycoproteins on RBC surface
Lipids
•
Hydrophobic: non-polar, water-hating
•
Do not cause osmotic pressure
•
Thermal insulator: conserve body heat
•
Shock absorber around vital organs
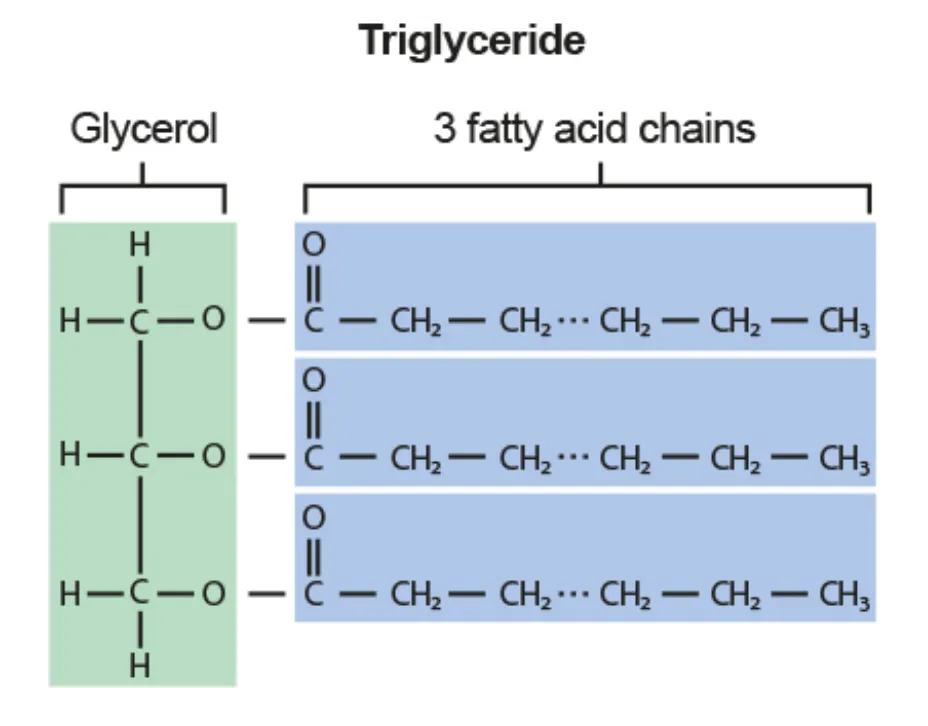
Triglyceride
Glycerol + fatty acids → triglyceride + water
•
Stored in adipose tissue: energy source + insulation
Fatty acid
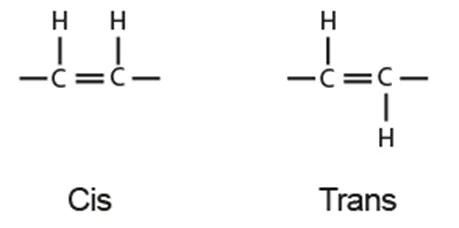
Saturated: all single bonds
Unsaturated:
•
Monounsaturated: 1 double bond
•
Polyunsaturated: > 2 double bonds
•
Cis/trans isomers
Phospholipid
Glycerol + 2 fatty acids + phosphate group
Hydrophilic head + hydrophobic tail → bilayer
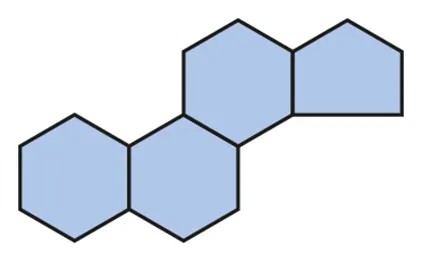
Steroid
Ring structure: pentagon + hexagon
Energy sources
Carbohydrates | Lipids |
Both are primary sources of energy | Both are primary sources of energy |
Energy per gram: 4 kcal/g
Soluble
Aerobic or anaerobic respiration
Short term storage: easier to transport = more accessible = quickly available | Energy per gram: 9 kcal/g
Non-soluble
Aerobic respiration only
Long-term storage |