Understanding points
Understanding points
C1.1.1 Enzymes as catalysts
C1.1.2 Role of enzymes in metabolism
C1.1.3 Anabolic and catabolic reactions
C1.1.4 Enzymes as globular proteins with an active site for catalysis
C1.1.5 Interactions between substrate and active site to allow induced-fit binding
C1.1.6 Role of molecular motion and substrate–active site collisions in enzyme catalysis
C1.1.7 Relationships between the structure of the active site, enzyme–substrate specificity and denaturation
C1.1.8 Effects of temperature, pH and substrate concentration on the rate of enzyme activity
C1.1.9 Measurements in enzyme-catalysed reactions
C1.1.10 Effect of enzymes on activation energy
C1.1.11 Intracellular and extracellular enzyme-catalysed reactions (HL only)
C1.1.12 Generation of heat energy by the reactions of metabolism (HL only)
C1.1.13 Cyclical and linear pathways in metabolism (HL only)
C1.1.14 Allosteric sites and non-competitive inhibition (HL only)
C1.1.15 Competitive inhibition as a consequence of an inhibitor binding reversibly to an active site (HL only)
C1.1.16 Regulation of metabolic pathways by feedback inhibition (HL only)
C1.1.17 Mechanism-based inhibition as a consequence of chemical changes to the active site caused by the irreversible binding of an inhibitor (HL only) |
Enzymes
•
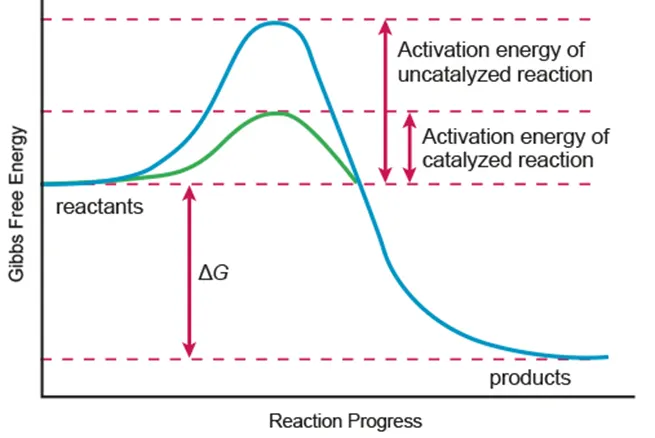
Biological catalysts that speed up the rate of reaction
•
Lower the activation energy (Eₐ) by providing alternative mechanism for the reaction
◦
Eₐ: minimum E required to initiate a reaction
•
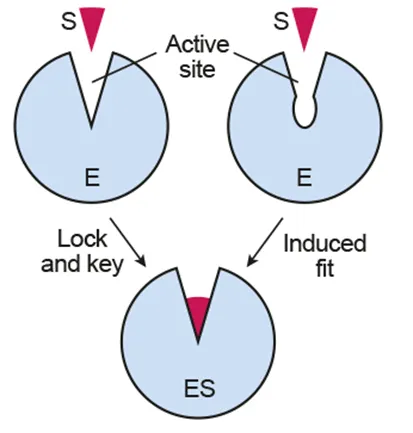
Active site: the specific binding site of a substrate
◦
Lock and key model: the enzyme has specificity for the substrate that fits in the active site
◦
Induced fit model: the active site changes its shape slightly so that substrate can fit
Anabolism vs Catabolism
Anabolism | Catabolism |
Requires energy
Simpler substances are transformed into more complex molecules
e.g. photosynthesis | Releases energy
Complex substances are broken down into simpler molecules
e.g. cell respiration |
Factors affecting enzyme activity
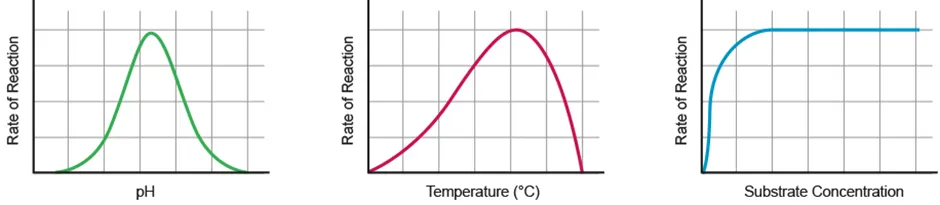
pH | - Optimum pH: fastest rate of reaction
- Strong acids / bases break bonds within enzymes, causing conformational change and denaturation → enzyme stops working |
Temperature | - Increase in temp. increases rate of reaction as the particles possess more KE, moving faster to have more collisions
- High temp. causes denaturation due to conformational change of active site
- Denatured enzymes become nonfunctional |
[Substrate] | - Increase in [substrate] increases rate of reaction as there is greater chance of collision between the substrate and enzyme
- Once all active sites are occupied, there is no further increase in rate |
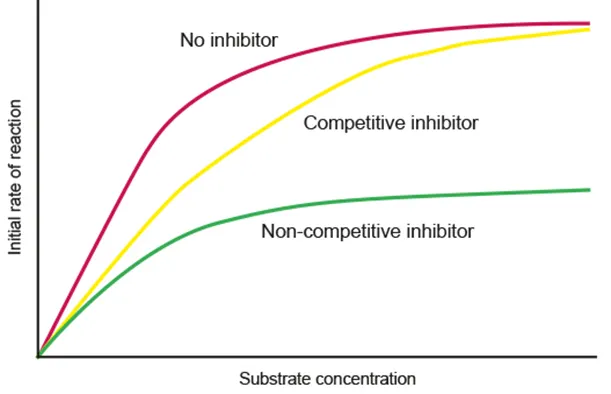
*(AHL) Enzyme inhibition
Competitive
Binds to the active site instead of the substrate
Reversible by increasing substrate concentration
Noncompetitive
Binds to allosteric site, causing a conformational change of the active site
Reversible
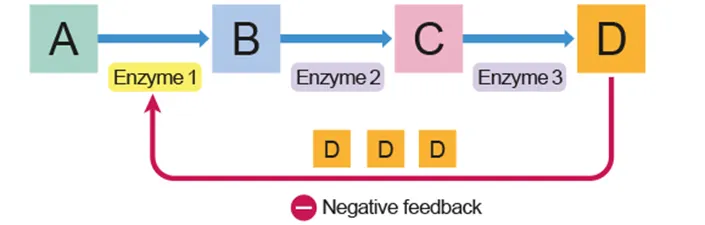
Feedback
A metabolic pathway is a series of reactions catalyzed by different enzymes
Lack of end product increases catalytic activity to produce more of final product
Excess accumulation of end product leads to non-competitive inhibition of the first catalytic reaction
Mechanism-based
Irreversible: bind covalently to the active site
e.g. penicillin inhibits transpeptidase in gram-positive bacteria