B.4.1 Basic Definitions
Internal Energy
Types of Systems
•
Closed system
•
Isolated system
Sign Convention
•
Clausius’ sign convention
B.4.1 First Law of Thermodynamics
First Law of Thermodynamics
Work Done by a Closed System
Change in Internal Energy
B.4.2 Entropy
Definition of Entropy
Entropy and Heat
B.4.3 Second Law of Thermodynamics
Second Law of thermodynamics
•
As a change of entropy
◦
•
Clausius statement:
◦
It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a lower-temperature body to a higher-temperature body.
B.4.3-1
•
Kelvin statement:
◦
It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce only a net amount of work.
B.4.3-2
Irreversible Processes
B.4.3-3
Reversible Processes
B.4.4 Thermodynamic Processes
Isovolumetric Process
B.4.4-1
Isobaric Process
B.4.4-2
Isothermal Process
B.4.4-3
Adiabatic Process
B.4.4-4
B.4.5 Heat Engines
Heat Engine Definition
Heat Engine Efficiency
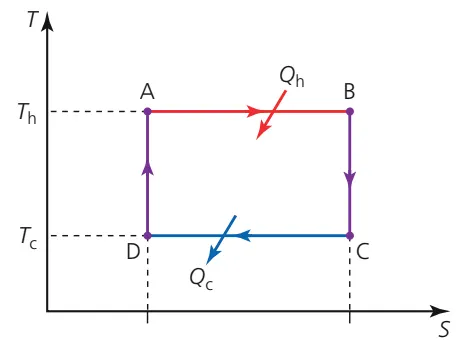
Carnot Cycle
B.4.5-1 Temperature-entropy diagram for the Carnot cycle