Understanding points
Understanding points
D2.3.1 Solvation with water as the solvent
D2.3.2 Water movement from less concentrated to more concentrated solutions
D2.3.3 Water movement by osmosis into or out of cells
D2.3.4 Changes due to water movement in plant tissue bathed in hypotonic and hypertonic solutions
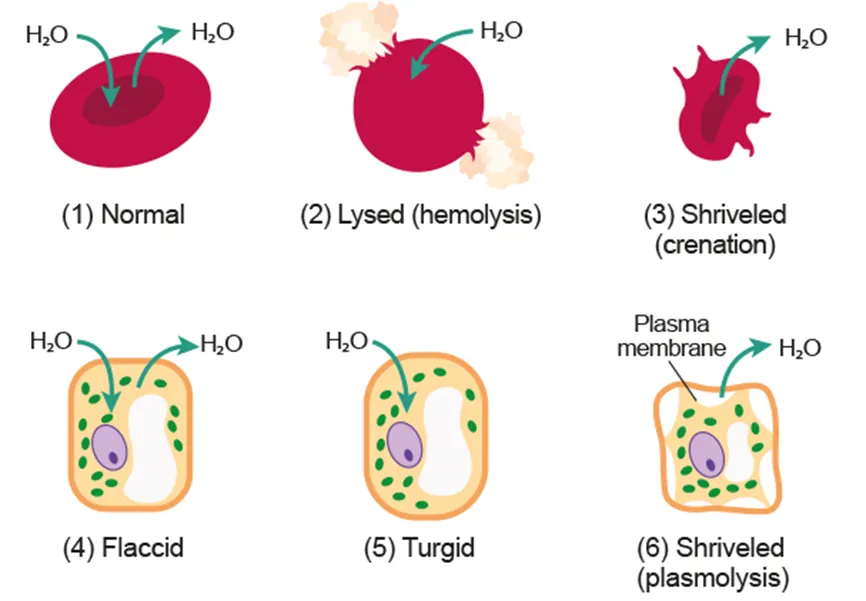
D2.3.5 Effects of water movement on cells that lack a cell wall
D2.3.6 Effects of water movement on cells with a cell wall
D2.3.7 Medical applications of isotonic solutions
D2.3.8 Water potential as the potential energy of water per unit volume (HL only)
D2.3.9 Movement of water from higher to lower water potential (HL only)
D2.3.10 Contributions of solute potential and pressure potential to the water potential of cells with walls (HL only)
D2.3.11 Water potential and water movements in plant tissue (HL only) |
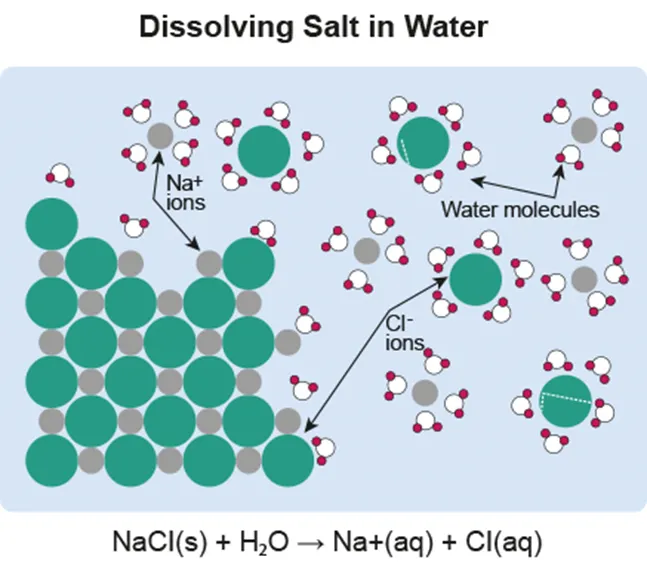
Solvation
•
The process of dissolving
•
Solvent: liquids that dissolve other substances
•
Solute: dissolved substances in solutions
Hypertonic | Higher [solute] |
Hypotonic | Lower [solute] |
Isotonic | Same [solute] |
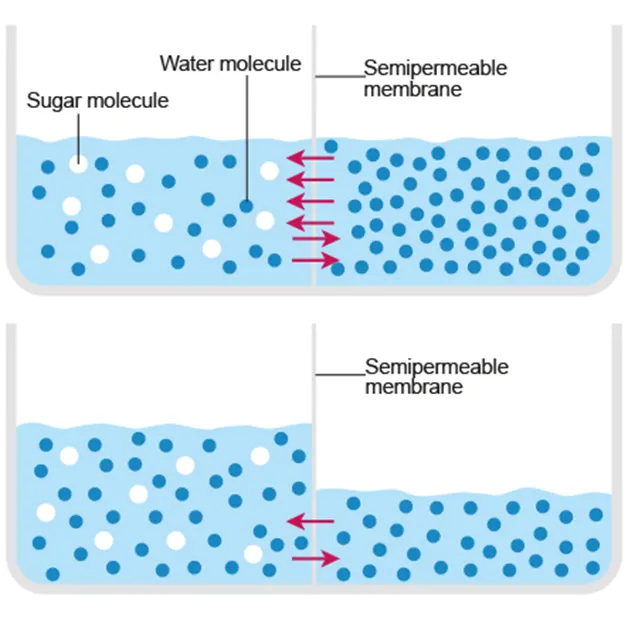
Osmosis
•
Diffusion of water molecules
•
Movement of water from high water potential to low water potential (hypotonic to hypertonic)
•
Intravenous fluids and organs used in transplants must be bathed in isotonic solution (0.9% NaCl) to prevent osmotic damage
*(AHL)
Water potential
•
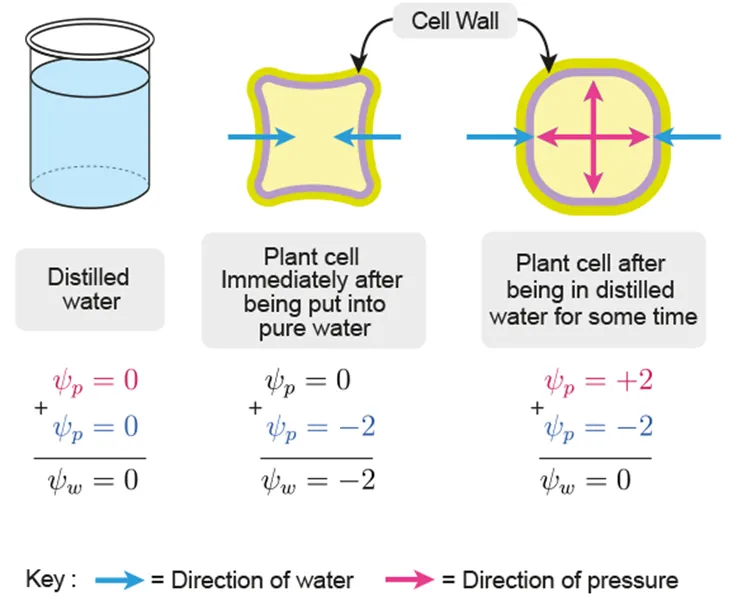
𝚿w: potential energy per unit volume (kPa)
Water 𝚿w = 𝚿s + 𝚿p | Pure water at standard pressure and 20 ℃ has 𝚿 = 0 (maximum value)
Water moves from higher to lower water potential because this minimizes its potential E |
Solute 𝚿s | The release of energy from bond formation during solvation
The more solutes dissolved, the more negative the 𝚿s |
Pressure 𝚿p | The higher the hydrostatic pressure, the higher the potential E of water
Plant cells have positive pressure potential (turgid)
Xylem vessels have negative pressure potential |
Water potential in plants
In pure water | Water moves into plant cell until it is fully turgid (𝚿w = 0) | |
In hypotonic solution | 𝚿s: plant cell < solution
→ 𝚿w of cell is lower | Water moves from solution into cell
→ 𝚿w of cell increases |
In hypertonic solution | 𝚿s: plant cell > solution
→ 𝚿w of solution is lower | Water moves from cell to solution
→ 𝚿w of cell decreases |