Understanding points
Understanding points
Structure 2.1.1—When metal atoms lose electrons, they form positive ions called cations.
When non-metal atoms gain electrons, they form negative ions called anions.
Structure 2.1.2—The ionic bond is formed by electrostatic attractions between oppositely charged ions.
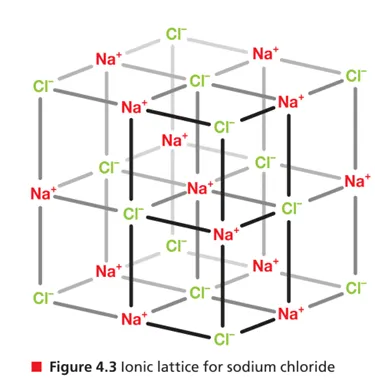
Structure 2.1.3—Ionic compounds exist as three-dimensional lattice structures, represented by
empirical formulas. |
Ionic bond
•
Transfer of electron(s) from a metal to a non-metal and the resulting electrostatic attraction between oppositely charged ions
•
Metals lose valence electrons to form positive ions (cations)
•
Non-metals gain electrons to form negative ions (anions)
Charge of cation and anion
Group | no. of Valence e- | Ionic charge |
1 | 1 | +1 |
2 | 2 | +2 |
13 | 3 | +3 |
14 | 4 | N/A - energy to transfer 4 e-s too large |
15 | 5 | -3 |
16 | 6 | -2 |
17 | 7 | -1 |
18 | 8 | N/A - no need to lose full valence shell |
Exceptions to the trend | Cationic charge |
Transition metals | multiple |
Pb | 2+ |
Sn | 2+ |
Ag | 1+ |
H | +1, -1 |
Polyatomic ions - charged covalent compound
OH- | hydroxide |
NH4+ | ammonium |
CO32- | carbonate |
SO42- / SO32- | sulfate / sulfite (sulfide S-2) |
PO43- / PO33- | phosphate / phosphite (phosphide P-3) |
NO3- / NO2- | nitrate / nitrite (nitride N-3) |
HCO3- | hydrogencarbonate/bicarbonate |
HSO4- | Hydrogensulfate |
CH3COO- | ethanoate |
Formula of ionic compound
•
Balance cation and anion charge to be equal - cross multiply cation and anion charges
NaCl | already balanced |
MgCl2 | 2 x 1 |
Al2O3 | 3 x 2 |
Lattice structure
•
Continuous, 3D network of repeating units of cations and anions
•
Because ionic bonds are nondirectional, each cation in the lattice attracts all the surrounding electrons → strong electrostatic attraction
•
Empirical formula expresses the simplest ratio of an ionic lattice structure
•
Coordination number: no. of ions that surround the opposition ion in lattice, for NaCl is 6
Physical properties of ionic compounds
High melting point | • Large energy needed to break strong electrostatic attraction between ions in lattice
• Higher ionic charge, increases electrostatic attraction between ions → higher melting point |
High solubility (in water) | • Dissolves in ionic/polar solvents due to its ability to attract the ions that make up the compound
• E.g. H₂O |
Electricity conductivity | • Electricity = flow of charge/ions
• Only as molten liquids or aqueous solutions as the ions are free to move |
Brittleness | • Shatter instead of being smoothly cut
• If a few ions are moved by force, the whole layer has to move |